Understanding the Legal Implications of Stem Cell Therapy in the United States
One question I often get asked by patients is about the potential of stem cell treatments for arthritis and rotator cuff tears.
There can be confusion about the regulations surrounding stem cell therapies in the United States, and some patients wonder if overseas treatment is necessary. It’s important to discuss these questions with a qualified medical professional.
Yes, some forms of stem cell therapy are legal and FDA-approved, while others are not. Understanding this complex landscape is important for patients considering this treatment option.
I’m Dr. Jorge Gonzalez, a specialist in Regenerative Orthopedics medicine and founder of the Institute of Regenerative Orthopedic and Sports Medicine (IROSM). In this article, I’ll guide you through the FDA regulations surrounding stem cell therapy and outline what you need to know before seeking treatment.
The FDA’s Role in Stem Cell Regulation
The Food and Drug Administration (FDA) plays a central role in ensuring the safety and effectiveness of stem cell therapies in the United States. Their goal is to protect patients and make sure that any stem cell treatment offered meets rigorous standards.
FDA-Approved Stem Cell Therapies
The FDA has approved several stem cell therapies for specific medical conditions. These include:
- Hematopoietic stem cell transplantation (HSCT) for blood disorders like leukemia and lymphoma.
- Mesenchymal stem cell (MSC) therapy for conditions like osteoarthritis and Crohn’s disease.
- Cord blood stem cell therapy for certain cancers and blood disorders.
Unapproved Stem Cell Therapies
Many clinics offer stem cell treatments that have not undergone the same level of FDA scrutiny. These treatments may not have been proven safe or effective through clinical trials. Some, like those utilizing unapproved sources of stem cells, may even be considered illegal.
The FDA actively works to limit the availability of these potentially risky therapies. At the moment, the FDA has made it clear that allogenic products like umbilical cord, placenta derived , amniotic cell, exosomes derived products are considered a drug and should not be used in the USA without a appropriate IND. An IND essentially means that the product is being investigated in a clinical trial being overseen by the FDA.
Protecting Yourself: What to Consider
- Consult a qualified doctor: Always discuss stem cell therapy with a knowledgeable medical professional like myself. You can find out more about my qualifications here. We can help you understand the regulations, weigh risks and benefits, and explore FDA-approved options.
- Visit the FDA website: Learn more about FDA guidelines and approved treatments at https://www.fda.gov/
- Beware of misleading claims: Be wary of clinics promising miracle cures with stem cells. If something sounds too good to be true, it probably is scam.
Finding Reliable Treatment Options
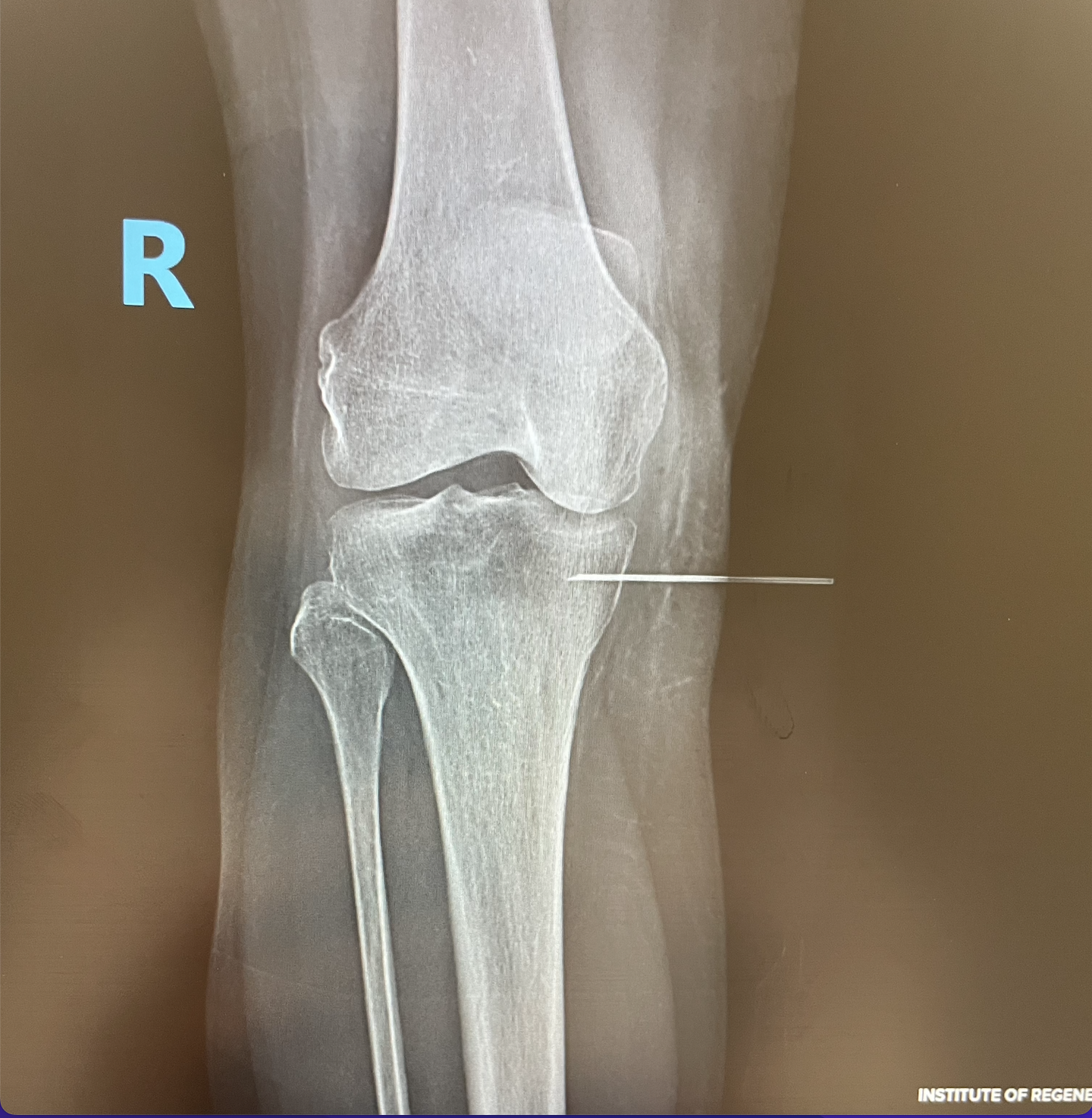
If you are considering stem cell therapy and how it can benefit you, thorough research and consultation with a specialist like Dr. Jorge Gonzalez of IROSM is essential. We can help you understand if your orthopedics or musculoskeletal condition can be treated with any of the regenerative orthopedics options available. If you are interested in a stem cell option currently not available in the USA, we can help you find current clinical trial(clinicaltrials.gov)
It’s important to understand your options and make informed decisions about your health. To learn more about the Regenerative Orthopedics options we offer,please give IROSM a call at (954) 751 6990 or visit our contact page to book an appointment.
At IROSM, we offer a variety of treatments to help as many patients as possible achieve the healthy and sustaining outcomes they deserve.
Disclaimer: This information is for educational purposes only and should not replace advice from your doctor.